Supported by a $1 million grant from the W.M. Keck Foundation, biologists Hui Zong and William Cresko are in the midst of an ambitious three-year project focused on genetic changes that occur in glioma, the deadliest form of brain cancer.
Genetic adaptation to different environments helped human beings and chimpanzees diverge from a common ancestor approximately six million years ago, resulting in the two separate species they are today.
This evolutionary process also happens at a cellular level in our bodies and may explain why cancer cells thrive and become deadly tumors.
Two UO researchers are committed to understanding this process, and their work may result in a fundamental impact on cancer research.
Supported by a $1 million grant from the W.M. Keck Foundation, biologists
Hui Zong and
William Cresko are in the midst of an ambitious three-year project focused on genetic changes that occur in glioma, the deadliest form of brain cancer.
This project was jump-started this year by a high-impact paper that identified the cellular origin of glioma, using an innovative mouse genetic model developed by a Zong-led team from the UO and Stanford University. The Oregon team is now working on the next important step: identifying the key genetic changes that lead to the glioma cancer cells.
Scientists have long suspected that dividing cancer cells can mutate and adapt in response to their surroundings within the body—just as organisms in the wild adapt over generations to changing environments. These genetic adaptations allow cancer cells to grow rapidly in number and strength, usually at the expense of healthy neighboring cells.
Unfortunately, cancer research is mostly limited to end-stage tumors since tumor cells are not readily visible at earlier stages when one uses conventional tools. These end-stage tumors often have many genetic changes, only a few of which are responsible for the cancer, so identifying causative changes in full tumors is very difficult.
The approach taken by Zong and Cresko is different from that of many cancer researchers, and solves this problem. Using a new cellular tool, they study cells far earlier in the process, starting with benign mutations and charting their path into full-fledged malignancies.
“The medical community has known for years that cancer is the consequence of many genetic and cellular alterations, rather than a sudden outburst of cell proliferation,” said Zong, assistant professor of biology in the
Institute of Molecular Biology. “The grant allows us to analyze cancer as an evolutionary logic.”
In addition to the potential for translating the discovery into clinical diagnosis and treatment, the UO Keck- funded technology should be able to determine the point of ignition in other cancers, Zong said.
This is a multidisciplinary effort that reflects “the deep history of integrative research at the University of Oregon,” said Cresko, an associate professor of biology in the
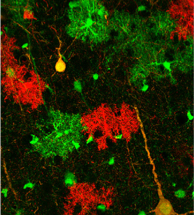
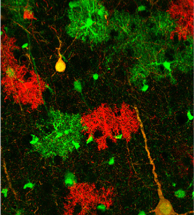
Institute of Ecology and Evolution. By combining cell-labeling approaches and a glioma mouse model developed in the Zong lab with genome-sequencing technologies and computational analysis equipment developed by the Cresko laboratory, the team will create tumor cells marked with green fluorescent proteins; normal cells are tagged with red ( image above).
The entire genomes of these cells will then be sequenced in the UO’s new High Throughput Sequencing Facility. By analyzing subtle genetic changes in the DNA of these marked cells, the researchers may provide a springboard to new interventions.
—ET






 Watch
Watch  Watch excerpts from the opera, coming soon to Eugene.
Watch excerpts from the opera, coming soon to Eugene. 

 Three’s a charm for a living memorial.
Three’s a charm for a living memorial.