Why drill for fossil fuels when an enormous, self-sustaining energy source is right over our heads? Green plants figured this out eons ago. We humans are just beginning to catch up.
Taking a cue from the Earth’s abundant plant life, UO chemist Shannon Boettcher is working on a method for using water to store energy from the sun. Given humanity’s growing energy demands, the looming threat of global warming and concerns over the safety of nuclear power, clean solar fuel is quickly gaining adherents.
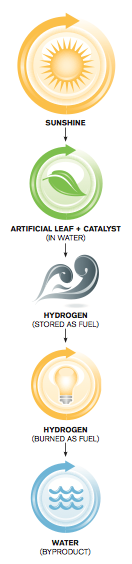
His work could have tremendous implications for the future of global energy production. What’s so startling is how clean the production of solar fuel can be: Sunlight is absorbed by a submerged semiconductor film, locally generating electrical energy; this energy, with help from a chemical catalyst, then splits water adjacent to the film into oxygen and hydrogen. The oxygen can be vented into the atmosphere, and the hydrogen can be stored for use as a fuel.
And the byproduct of burning hydrogen fuel? Water.
“The idea is to use the energy in sunlight to split water into hydrogen and oxygen,” said Boettcher, an assistant professor of inorganic and materials chemistry and a signature researcher with the Oregon Nanoscience and Microtechnologies Institute. “This is a way to store the energy from sunlight in a chemical fuel, much like plants do using photosynthesis.”
In photosynthesis, chlorophyll captures sunlight and uses it to split water in a plant’s leaves into oxygen and hydrogen ions; the hydrogen ions combine with electrons and carbon dioxide to form sugar. The energy of the reaction is stored in the sugar’s chemical bonds for later use by the plant—and, later still, by plant- eating humans.
Boettcher’s task is to synthesize new materials—in essence, to create artificial leaves—that mimic the reaction- spurring role that chlorophyll plays in photosynthesis. But instead of producing sugar as plants do, these man-made leaves would produce hydrogen fuel, which burns cleanly and could be used to power cars, create heat or generate electricity. His research could lead to new, more efficient techniques for large-scale solar energy harvesting and storage.
Traditional solar cells, which convert sunlight into electricity, are highly efficient but are expensive, require a great deal of energy to produce and work only when the sun is shining, said Boettcher. Plus, storing electricity from solar cells is cumbersome and costly. Hydrogen is a more versatile end-product: It can be stored as a compressed gas and burned like natural gas to generate heat that can drive a turbine, be recombined with oxygen in a fuel cell to create electricity or be combined with carbon dioxide to form methanol (a gasoline substitute).
“Many researchers are working on ways to store hydrogen in less space so that we could better use it as a transportation fuel,” said Boettcher.
But there is still much work to be done. First, a stable, efficient sunlight- collecting material—the outer surface of the artificial leaf—must be developed. Boettcher and his colleagues are looking at semiconductors such as tungsten oxide and iron sulfide. Second, they need to develop an abundant, inexpensive catalyst to drive the water-splitting reaction, like nature’s catalyst in green leaves.
Like a fuel production cycle that starts with water and ends with water, Boettcher’s academic life has come full circle: After graduating from the UO in 2003 with a bachelor’s degree in chemistry, he completed a PhD in inorganic materials chemistry at the University of California at Santa Barbara and held a postdoctoral position at the California Institute of Technology; he then joined the UO faculty in March 2010. He was recently selected as one of only 18 scientists worldwide to be named to the 2011 class of DuPont Young Professors.
—Eric Tucker




 Watch
Watch  Watch excerpts from the opera, coming soon to Eugene.
Watch excerpts from the opera, coming soon to Eugene. 

 Three’s a charm for a living memorial.
Three’s a charm for a living memorial.