Go With Your Gut
We are not alone.
Not because of visitors from other planets—these creatures aren’t even visitors. Most of them have been here longer than us.
What’s keeping us company—in ways we are just beginning to comprehend—are microscopic beings. Untold legions of them. Bacteria, fungi, viruses.
Microorganisms.
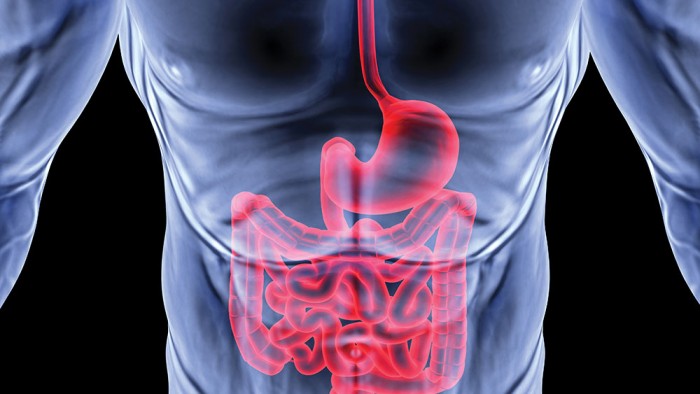
They inhabit the air we breathe, the ground we walk on and everything we touch; they inhabit us. We are each host to a massive community of microbes, numbering in the trillions. Most of them make their home in, of all places, the digestive tract—and UO scientists are on the cutting edge of research that suggests these microbial companions do a lot more than just process what we had for lunch.
For example, not long ago it was believed that peptic ulcers were caused by worry or spicy food. Now we know that one of the most common sources of ulcers is an infection by the bacterium Helicobacter pylori, which in some people damages the coating that protects the lining of the stomach.
As scientists at the UO and elsewhere focus on the human gut, they’re finding intriguing signs that the complex world of microbes there might play an important role not only in intestinal health but also the way our brains develop and how conditions such as autism emerge.
“Recent science has shown that some of these microbes interact with us in very important ways: altering how we develop as children, influencing our immune system, affecting our digestion, protecting our health, perhaps even changing our behavior,” said Brendan Bohannan, a UO biology professor. “Science is just beginning to understand these interactions, but the potential for this new understanding to lead to innovations in human health and well-being is huge.”
Only 10 percent “us”
Consider this: The human body has an estimated 100 trillion cells, some sources say, but only 10 trillion or so are “us”—that is, our blood cells, our muscle cells, and so forth. The other 90 percent are joyriding microorganisms—yes, 90 trillion critters cohabiting each of our bodies, a good portion of them classified as bacteria.
Of all the nooks and crannies in a human being where these tiny organisms hang out, no place says home like the gut. The human gut is awash in trillions of microscopic organisms, harboring a larger population than any other part of the body.
“The human gut is special because it’s so densely populated,” said Karen Guillemin, a UO biology professor. “The challenge for the field right now is to understand how these bacteria affect both our development and our health.”
Guillemin has made a career of studying the myriad microorganisms found in living animals. Lately she’s been focused on microbes in the digestive systems of vertebrates, with a particular eye toward understanding how what she observes in animals—specifically the zebra fish—gives insight into the human gut microbiome. (A number of UO researchers, in fact, are at the forefront in this area.)
The UO has pioneered the use of zebra fish as a model organism. Unlike other common research organisms such as fruit flies and nematode worms, zebra fish have backbones, and thus are remarkably similar to human beings in both genetic makeup and embryonic development. They also reproduce quickly, allowing Guillemin and others to raise zebra fish with no gut bacteria and compare them to unaltered zebra fish to better understand how bacteria affect development.
They can also restore gut bacteria to zebra fish to see whether certain bacteria are helpful, and in what way. Of course, these kinds of tests aren’t possible in humans.
The strength of the research program on zebra fish is one reason Guillemin came to the University of Oregon. Now she’s at the forefront of an area with huge promise—but also challenging obstacles.
“We’re just scratching the surface of what the biological capacity of this system might be,” Guillemin said. “We’re at an exciting stage in our knowledge, but it’s an early stage.”
Breast milk feeds bacteria
The wide-ranging involvement of microbes in health and human biology is an emerging field. The involvement of H. pylori in causing ulcers has only been known since the 1990s, and some of the most surprising revelations have taken place within the last five years.
Take the example of complex sugars commonly found in breast milk. Researchers discovered that these sugars couldn’t be broken down and used by the infant, raising questions about their roles.
It turns out that gut bacteria use these sugars to grow. Human babies are born without any microbes in their digestive tracts, and the sugars help them form their own microbe communities, or “microbiomes.” The discovery will influence how we look at infant nutrition, Guillemin said.
Research also suggests that changes in the gut microbe community could play a role in intestinal diseases and that changing the makeup of the bacterial community could treat some infections.
For example, doctors recently discovered that bacteria from the gut of a healthy person can be used—via a procedure called a fecal transplant—to treat people infected with a particularly stubborn and hard-to-treat bug that causes diarrhea and abdominal pain.
People who have been treated with antibio-tics are at risk for this infection because this pathogen can spread when the normal gut bacteria have been weakened by the antibiotics. But transplanting gut bacteria taken from the fecal matter of a healthy person appears to restore a normal, healthy gut microbiome that can fend off the infection. Clinicians speculate that the same treatment could be used to help people with irritable bowel syndrome, colitis, constipation and other intestinal conditions.
But Guillemin is cautious. “We don’t yet know how to precisely manipulate people’s gut microbiota to restore them to a healthy state,” she said. “Understanding how to engineer gut microbial communities is something we’re trying to do in our animal models.”
Gut bugs and the brain
Recently, a team of UO scientists focused on the potential effects of gut bacteria on the brain won $330,000 from the National Institute of Mental Health. Another $1.3 million grant is expected to follow.
Biologists Judith Eisen and Philip Washbourne will lead an exploration of the role that gut microbes might play in disorders such as autism, intellectual disabilities and schizophrenia.
With support from Guillemin and Cristopher Niell, an assistant professor of biology, the scientists will look at how gut microbes might affect the growth of synapses, the relay points that carry signals in the brain. It’s something Eisen has been thinking about since 2009, when few others saw any potential in the field.
“Everybody thought it was a crazy idea,” she said.
Preliminary evidence suggests that when animals are raised in an environment that prevents the development of a gut microbiome, genes that control synapse formation are affected. These same genes also appear to be involved in disorders involving neurological development, such as autism, so it’s possible that the lack of certain microbes in the gut could be tied to developmental disorders.
“It’s mind-blowing to think that the microbiome is so important in so many ways, and yet we really didn’t understand this until recently,” said Eisen, a neurobiologist who studies brain and spinal cord development. “You can look at it in another way, though. We evolved with these organisms, so it’s entirely unsurprising that we should have this incredibly intimate relationship.”
—Greg Bolt


 Twitter
Twitter Facebook
Facebook Forward
Forward